Are you grappling with the intricate demands of pharma compliance training? In today’s stringent regulatory environment, biopharmaceutical companies must ensure that scientists and staff meet compliance standards without fail. This post will examine how Totara Learn technology can simplify your compliance journey, focusing on tailored solutions for pharmaceutical regulations and enhancing data integrity through case management systems. Discover the benefits of automating compliance tracking and engaging employees in training, ultimately leading to measurable success. You’ll learn how Totara Learn not only addresses these challenges but also elevates your organization’s compliance policy to new heights.
Understanding the Challenges of Pharma Compliance Training
In the dynamic field of pharmaceuticals, you face numerous compliance challenges, from quality management to meticulous clinical trial oversight. To safeguard efficiency and precision, we’ll explore common compliance issues, underscore the need for robust training systems, and dissect the intricate regulatory landscape. As we assess these concerns, unveiling the utility of a pharmaceutical assistant course powered by analytics emerges, ensuring that you’re equipped to satisfy the stringent demands of the industry.
Identifying Common Compliance Issues in Pharmaceuticals
You understand that navigating the complexities of pharmaceutical manufacturing demands meticulous attention to detail, especially when it comes to compliance. The chemistry behind each formula must be precisely maintained, while rigorous verification and validation are crucial in fostering trust and ensuring patient safety. Challenges often arise in maintaining consistent quality and documentation standards, which are crucial for passing inspections and upholding accountability.
To remain compliant and competitive, you recognize the imperativeness of adherence to these standards. You are acutely aware that lapses in inspection readiness or incomplete handling of verification and validation can lead to significant repercussions, not just regulatory but also in terms of your organization’s reputation. Your commitment to the integrity of pharmaceutical practices entails a staunch dedication to thorough compliance training, underscoring the necessity of advanced learning management systems like Totara Learn.
Understanding the Need for Effective Training Solutions
Your striving for mastery in the handling of active pharmaceutical ingredients calls for enhanced intelligence and visibility throughout your processes. Pertinent professional development opportunities, such as pharmacy technician training online, are imperative to equip your team with the knowledge to navigate the evolving compliance landscape. Totara Learn technology provides these solutions, fostering a culture of continuous learning and regulatory adherence within your organization.
To address this, you need a training solution that transcends traditional methods, offering flexibility and customization to meet individual and organizational goals. Totara Learn delivers an unprecedented level of control over your training environment, empowering you to create specialized compliance courses defined by engaging content and real-world applications, directly aligning professional development with business outcomes.
Analyzing Regulatory Requirements for Pharma Companies
In the realm of pharmaceuticals, your paramount concern is upholding patient safety, and this hinges on strict adherence to regulatory requirements. Each pharma company must navigate an intricate web of guidelines that dictate the development, testing, and distribution of medications. Boasting intuitive educational tools, Totara Learn assists in sculpting your team’s skills so that every piece of legislation is not only understood but fully integrated into your daily operations, thus cementing customer trust in your commitment to excellence.
Staying abreast of regulatory changes calls for continuous education and a workforce proficient in the latest compliance practices. Totara Learn technology stands as your ally, offering a dynamic platform where customized learning equips your staff with the necessary expertise. With real-life examples and actionable insights woven into each course, your team will learn to flawlessly apply the most current compliance standards, reinforcing the backbone of patient safety and service quality that your customers rely on.
Compliance hurdles in the pharmaceutical industry are real and robust. Totara Learn smooths the path, standing by to simplify your strides.
Implementing Totara Learn for Streamlined Compliance
Embracing Totara Learn technology, you can streamline compliance management within your pharmaceutical operations, integrating seamlessly into existing training systems. You’ll configure compliance modules that address the intricacies of drug discovery, packaging, and labeling and optimize your workflow through innovative virtual training. These enhancements in your technology suite ensure a transformative impact on your organization’s learning structure, equipping your team with the resources necessary for watertight compliance and regulatory mastery.
Setting Up Totara Learn for Compliance Management
Your role in the healthcare industry requires a formidable grasp of regulatory compliance, and the right system is central to this need. By investing in Totara Learn, you’re equipping your organization with a robust pharmaceutical tech certification platform. This investment indicates your proactive stance in meeting the complex requirements of the industry with a structured, scalable learning solution.
As you adopt Totara Learn’s system, you will notice how seamlessly it supports the regulations dictating pharmaceutical practices. Comprehensive training modules tailored to your specific needs will cultivate a knowledgeable workforce, minimizing risk and reinforcing your commitment to industry standards. This strategic move not only streamlines your compliance management but also signifies your dedication to excellence within the pharmaceutical realm.
Integrating Totara Learn With Existing Training Systems
As you seek to bolster your pharmaceutical company’s training framework, Totara Learn emerges as the solution for seamless integration with your existing systems. It serves as a bridge between drug development processes and comprehensive regulation knowledge, all the while enhancing communication across your team. This integration aims to streamline your entire compliance infrastructure, positioning you for success in an industry where precision and adherence to standards are non-negotiable.
Embracing Totara Learn within your operations means effectively managing the demands of outsourcing and internal training in the pharmaceutical industry. Through the tailored configuration of Totara’s robust platform, you can ensure that your staff is not only up-to-date with the latest compliance requirements but also skilled in applying this knowledge in practice. The strategic alignment of existing resources with Totara’s capabilities justifies your investment and fortifies your commitment to unrivalled quality and safety standards.
Configuring Compliance Modules Within Totara Learn
As a chief compliance officer in the pharmaceutical sector, you recognize that tailoring compliance modules within Totara Learn to reflect the peculiarities of pharmaceutical formulation is key. Crafting these modules carefully, you secure practical employment within your organization of structured compliance pathways, ensuring all facets of research and development adhere to the requisite statutes and best practices.
Within Totara Learn, the power is yours to build and configure compliance modules that serve as cornerstones for your organization’s regulatory training initiatives. By integrating hands-on scenarios and empirical data, you deliver real-world relevance to your team’s learning experience, directly enhancing their capability to apply compliance knowledge to the actual work they perform daily.
Streamlined compliance is a start. But tailor-made solutions for pharmaceutical regulations take precision and knowledge to the next level.
Customizing Totara Learn for Pharmaceutical Regulations
To ensure your training aligns with the ever-evolving standards in medicine and biotechnology, Totara Learn can be expertly customized. Adapting course content to meet regulatory standards, you can design learning paths that fully prepare pharmacy technicians and laboratory personnel. Integrating GxP—good manufacturing practice and more into training modules ensures staff are proficient in the critical elements of compliance. This focus on customization facilitates a compliant, knowledgeable, and skilled workforce ready to meet the rigors of the pharmaceutical industry.
Adapting Course Content to Meet Regulatory Standards
As you strive to uphold patient safety and meet regulatory benchmarks, customizing course content within Totara Learn becomes pivotal. You need a comprehensive understanding of pharmacology, and Totara Learn facilitates this by allowing you to embed the latest information, automate knowledge assessments, and update course materials in line with changing regulations, ensuring your team’s expertise remains at the industry’s cutting edge.
Integrating automation into your learning system supports a seamless transition between learning modules and practical application, bridging gaps in knowledge and understanding. By customizing Totara Learn to reflect the complexities of pharmaceutical regulations, you empower your staff with the proficiency to transcend the typical compliance challenges, fostering a culture where patient well-being is paramount.
Designing Customized Learning Paths for Compliance Training
Your pursuit of compliance mastery as a pharmaceutical technician begins with personalized learning paths within Totara Learn. Skillfully designed courses tailored for law graduates fold into a cohesive pharmaceutical technician course, bolstered by an extensive database and streamlined management tools. This approach not only forges a clear route to certification but also anchors your understanding of complex regulations.
By engaging in custom training modules on Totara Learn, you directly address the nuanced aspects of pharmaceutical compliance. The platform’s flexible design allows you to immerse yourself in real-world scenarios that bridge the gap between theoretical knowledge and practical application, ensuring you are well-equipped to navigate the intricacies of industry standards and patient safety protocols.
Incorporating GxP Requirements Into Training Modules
Integrating GxP requirements into your training regimen within Totara Learn is essential to enhancing productivity and ensuring that all procedures meet the highest industry standards. As you work towards a postgraduate diploma or engineer further developments in pharmaceuticals, this platform’s capacity to align learning with these stringent regulations is invaluable. Totara’s adaptability ensures that your expertise remains robust amidst the rapidly advancing field, backed by the support of artificial intelligence to personalize the learning experience.
Totara Learn empowers you to incorporate a comprehensive GxP framework into educational courses, underpinning all aspects of product life-cycle management from laboratory to patient. By creating training modules that reflect these exhaustive requirements, you ensure that learning extends beyond theoretical knowledge, equipping your team with the practical tools to optimize efficiency and navigate regulatory landscapes successfully. This approach to professional development through Totara Learn is pivotal for maintaining the integrity and safety that your role in healthcare demands.
The precision of custom configurations sets the stage. Now, Totara Learn’s automation brings perpetual vigilance to your compliance needs.
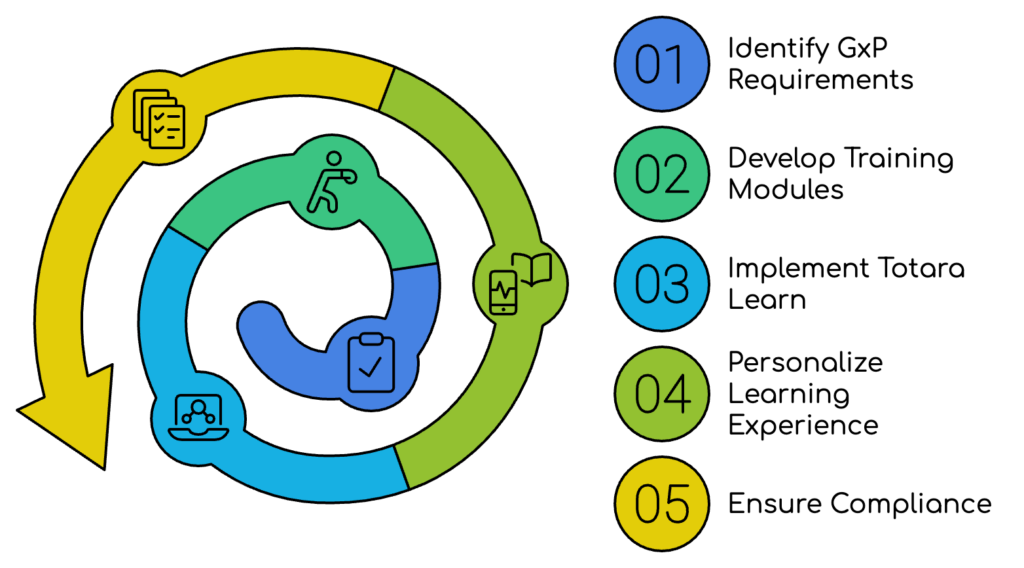
Illustration of Totara Learn’s GxP-integrated training modules, showcasing personalized compliance pathways and automation for enhanced regulatory adherence in pharmaceutical and healthcare training
Automating Compliance Tracking With Totara Learn
In your quest to maintain compliance within the pharmaceutical landscape, Totara Learn provides powerful technology to automate compliance tracking. With this system, you’re capable of enabling automated notifications for upcoming compliance deadlines, generating real-time reports and dashboards to monitor regulatory adherence, and tracking employee progress in certifications relevant to orphan drugs, medical devices, and risk management. Each feature is designed to simplify the complexities of your pharmaceutical business, ensuring that compliance is not just achieved but sustained.
Enabling Automated Notifications for Compliance Deadlines
You understand the critical nature of compliance deadlines in the pharmaceutical industry, and with Totara Learn, you gain a comprehensive system that simplifies these demands. The platform is adept at sending automated notifications to ensure deadlines are met, preventing oversights that could lead to data breaches or regulatory fines. This proactive approach to compliance management streamlines your experience, facilitating the safeguarding of sensitive data and maintaining a spotless compliance register.
With Totara Learn, you can channel your focus on innovation and progress, knowing that the compliance framework is reliably managed by automated systems. Notifications serve as timely reminders for critical document submission, training updates, and other compliance-related tasks, minimizing the risk of a data breach. This functionality empowers your team to be adept at managing regulatory data, ensuring each member is registered and informed in a timely manner, and driving compliance as a seamless and integral part of your daily operations.
Generating Real-Time Compliance Reports and Dashboards
As part of your commitment to excellence in healthcare, you can leverage Totara Learn to generate real-time compliance reports and dashboards. This dynamic feature enables you to conduct thorough risk assessments and monitor adherence to clinical research protocols instantly. By having immediate access to actionable data, you maintain a firm grasp on the critical metrics that indicate your organization’s regulatory health.
Utilizing Totara Learn’s bespoke reporting tools, you empower your team to identify areas in need of attention and foster a culture of proactive management in your healthcare operations. The ability to customize reports and dashboards specific to your requirements ensures that you stay ahead, securing not just compliance but also the safety and efficacy that is paramount in pharmaceutical endeavours.
Tracking Employee Progress and Certification Status
With Totara Learn, you gain the finesse to monitor employee progress towards meeting quality control benchmarks essential in your role. Totara Learn automates the tracking process, providing real-time visibility into the certification status of team members engaged in an assistant pharmacy course. Emphasize transparency in workforce development, aligning onboarding practices with industry standards.
You’ll appreciate the simplicity with which Totara Learn keeps you apprised of staff proficiency levels, simplifying the complexity of compliance management. This acts as a pivotal tool for HR managers, offering clarity and actionable details on training outcomes and ensuring that everyone remains ahead in their compliance knowledge and skills—a testament to the platform’s utility in fostering a culture of continuous improvement.
Tracking compliance is only the beginning. Now, consider how engaging your team makes all the difference.
Boosting Employee Engagement in Compliance Training
In your pursuit of pharmaceutical excellence, engaging your team in compliance training is critical for mastering regulations like the General Data Protection Regulation and ensuring pharmacovigilance, but this often requires more than just routine courses. To foster confidence and thorough understanding, Totara Learn integrates interactive content, gamification, and mobile accessibility—key features that invigorate learning experiences and encourage consistency, even during a background check. Let’s explore how these tools transform the mundane into interactive learning journeys for every pharmacy professional.
Utilizing Interactive Content to Increase Engagement
Engaging you in the compliance training process is paramount, and Totara Learn excels as a pivotal tool for achieving this through interactive content. By involving you in scenarios that accurately represent governance challenges and supply chain complexities, the system enhances your understanding of Food and Drug Administration regulations. This approach not only informs but also immerses you in the practical application of compliance protocols, solidifying your grasp of industry expectations.
Incorporating the power of interactive learning, Totara Learn transforms your training experience by simulating real-world compliance tasks. You’ll find that these engaging modules elevate your capacity to internalize crucial governance principles and apply them effectively within your pharmaceutical role. As a result, you are better prepared to navigate the intricacies of the supply chain, ensuring safety and efficacy from development to delivery.
Implementing Gamification in Compliance Courses
You’ll find that implementing gamification in compliance courses through Totara Learn transforms the mandatory task of pharmaceutical technician certification into an engaging experience. This approach, applying game-design elements to the coursework, incentivizes you to excel in understanding the complexities of cleanroom protocols and manufacturing compliance.
Engage in a pharmacy assistant online course that employs gamification and watch as it redefines the learning process within the industry, making it interactive and rewarding. By integrating gamification, Totara Learn helps you master the regulations and procedures essential to your role, enhancing retention and application of the knowledge in practical settings.
Providing Mobile Access to Training Materials
Providing mobile access to training materials means you’ll have the flexibility to engage with critical compliance content at any juncture in the product lifecycle, whether in the lab or on the move. This accessibility allows you as an expert in medicinal chemistry to integrate learning seamlessly into your daily routine, ensuring you’re up-to-date with the latest compliance protocols and version control methodologies critical to your company’s success.
Totara Learn technology enhances your expertise by offering mobile solutions that keep you connected to the latest training materials. This level of convenience ensures you’re never behind on the evolving landscape of pharmaceutical regulations, making it simpler to maintain a keen understanding of intricate compliance specifics no matter where you are, directly contributing to the high standards upheld by your organization.
Elevating employee commitment to compliance is just the beginning. Now, let us shift focus to the tangible outcomes, scrutinized through Totara Learn built-in Analytics.
Measuring Compliance Success With Totara Learn Analytics
Mastering pharmaceutical compliance hinges on the power of data-driven insights. Totara Learn Analytics serves as your dashboard to interpret vital learning data, benchmark compliance performance across various departments, and make precise training adjustments based on data revelations. In the complex landscape of medicine and vaccine science, leveraging these analytics allows you to conduct root cause analysis and refine strategies to enhance compliance adherence.
Interpreting Learning Analytics for Improvement
When you harness the rich, data-driven feedback from Totara Learn’s analytics, you are preparing to elevate your compliance management system to new levels of efficacy. By analyzing key metrics and trends, you can identify the strengths and weaknesses in your pharma compliance training programs, allowing you to design targeted interventions that continuously enhance your team’s proficiency with complex regulations like the Health Insurance Portability and Accountability Act (HIPAA).
Understanding that improvement is a constant pursuit in compliance, you can leverage Totara Learn analytics for regular audit preparations, ensuring your practices not only meet but exceed industry standards. This technological support aids you in evolving your strategies proactively, sending timely email reminders for crucial compliance updates, and streamlining your approach to stay ahead of the pharmaceutical compliance curve.
Benchmarking Compliance Performance Across Departments
In your role as a leader within the pharmaceutical industry, you understand that cohesive compliance across departments is key to ensuring drug safety and effective aseptic processing. By leveraging Totara Learn analytics, you can benchmark performance, identifying areas where specific departments excel or require further development. This tailored analysis guides your teams toward a unified standard of excellence, which is crucial in an environment where every detail can impact patient health.
Totara Learn’s robust analytics enable you to scrutinize development processes department by department, providing a clear view of how safety protocols and compliance measures are upheld throughout your company. With this insight, you can foster leadership that is well-informed and proactive, driving departments towards achieving benchmarked goals that enhance overall compliance, thus strengthening the safety and efficacy of your pharmaceutical outputs.
Adjusting Training Based on Data Insights
When you employ Totara Learn’s analytics, you gain the insight to finely tune your pharmaceutical training endeavors. Specifically, if data reflects gaps in areas such as regulatory affairs or microbiology, your Totara courseware can be swiftly adjusted. This ensures that your team’s expertise—be it for a diploma in pharmaceutical microbiology or a technician pharmacy course—is always aligned with the latest industry standards, fully preparing them for the precision required in their roles.
Your continuous engagement with Totara Learn analytics allows for a proactive refinement of training modules based on real-world performance data. Should the insights reveal an emerging need within your organization, such as a deeper understanding of microbiological contamination control, you can promptly integrate this focus into your training. This responsive approach secures a robust, data-driven educational foundation for your staff, reinforcing the imperative compliance that underlies their essential work in pharmaceuticals.
Conclusion
In the intricate world of pharmaceutical compliance, mastering regulations is pivotal, and Totara Learn emerges as a sophisticated ally, providing a dynamic platform for creating potent, personalized training experiences. Through its advanced capabilities in compliance tracking, regulatory content adaptation, and GxP-aligned interactive learning modules, Totara Learn enables pharmaceutical organizations to streamline and enhance compliance processes. The platform’s powerful analytics allow leaders to benchmark, assess, and adapt training in real-time, supporting continuous improvement and ensuring the pharmaceutical industry upholds the highest safety and efficacy standards. By transforming compliance into a strategic advantage, Totara Learn fosters a culture of proficiency and vigilance—cornerstones of patient safety and pharmaceutical excellence.
Ready to elevate compliance training in your pharmaceutical organization? Reach out to Markanyx, the exclusive Totara provider in Canada, for a complimentary assessment and demo. Discover how Totara Learn can empower your team to achieve unmatched standards in safety, compliance, and quality.





